

WHY PRP?
AN EFFECTIVE BIOACTIVE THERAPY
Platelet-Rich Plasma (PRP) is a biological product obtained from the patient own blood (autologous) which contains a platelet concentration above normal basal value. Platelets and plasma of PRP contain a wide amount of growth factors (GFs) and other biomolecules that activate and accelerate the processes of repair and regeneration of tissues where they are administered.
EXPECTED EFFECTS
Blocks the production of inflammatory cytokines while promoting the synthesis of anti-inflammatory cytokines.1,2
Induces stem cell chemotaxis and proliferation in the area of application, replacing the damaged cells and stimulating extracellular matrix production.3,4
Promotes neovascularization in injured and fibrotic tissues.5
Induces a cell-based endocannabinoid response in inflammatory conditions, decreasing pain.6
Positively modifies cellular metabolism activating anabolic pathways for formation of new tissues, while also inhibiting its degradation2.
Slows the growth of bacteria and decreases the possibility of infections.9
APPLICATIONS
CHRONIC:
Osteoarthritis:
- II, III - intra-articular (IA) PRP.
- III, IV - intraosseous & IA PRP.
Tendinopathies (enthesitis or tear)
Others Plantar fasciitis, degenerative meniscal tear, post-meniscectomy syndrome, degenerative disc disease, fibrotic and painful scars.
ACUTE
Muscle, tendon and ligament tears.
BIBLIOGRAPHY
1. Andia et al. Nat Rev Rheumatol. 2013; (12):721-30.
2. Moussa et al. Exp Cell Res. 2017; 352(1):146-156.
3. Sclafani et al. Arch Facial Plast Surg, 2012;14(2):132-6.
4. Schär MO et al. Clin Orthop Relat Res. 2015; 473(5):1635-43.
5. Kakudo et al. Med Mol Morphol. 2014; 47(2):83-9.
6. Descalzi et al. Tissue Eng Part A. 2013;19(19-20):2120-9.
7. Sundman et al. AJSM. 2014; 42(1):35-41.
8. Sakata et al. AJMS. 2015; 43(6):1467-73.
9. Li et al. J Vis Exp 2013; 9(74).
10. Dai et al. Arthroscopy. 2017;33(3):659-6702017.
11. Shen et al. J Orthop Surg Res,. 2017; 23;12(1):16.
12. Chen et al. AJSM, 2017.
13. Ghaffarpasand F et al. Bull Emerg Trauma. 2016; 4(3):134-40.
14. Magalon et al. BMJ Open Sport Exerc Med. 2016; 4;2(1):e000060
HY-tissue PRP: A UNIQUE TECHNOLOGY
USER FRIENDLY
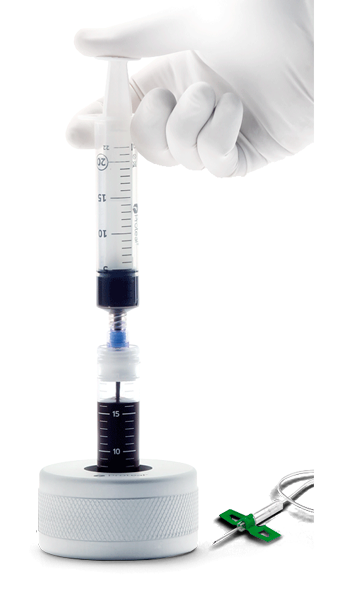
Hy-tissue PRP 20/50 are easy to use and have simple step-by-step

TOTAL PROTECTION
CLOSED SYSTEM Blood and plasma transfer through a swabable luer valve, designed to fill, hold and release controlled amounts of fluids on demand.
USER PROTECTION To ensure user safety at any time during handling, the kit has:
- Safety mechanism to minimize the risk of a needlestick injury
- Flexible cannulas

For all therapeutic needs in outpatients or in the operating room:
2 KITS AVAILABLE:
- Hy-tissue PRP 20
- Hy-tissue PRP 50
2 TYPES OF PRP:
- VERY PURE PRP
- LEUCO-RICH PRP

2 Individual sterile kits in the same pack, with all components for:
EXTRACTION KIT
- Collection of anticoagulated blood
PRP KIT
- PRP processing, selection and activation
CELL FRIENDLY
 PROTECTIVE SURFACE
PROTECTIVE SURFACE
 Devices made of anti-electrostatic medical grade polymer with a nano-polished and coated surface to prevent platelets adhesion, which increase platelet recovery.
Devices made of anti-electrostatic medical grade polymer with a nano-polished and coated surface to prevent platelets adhesion, which increase platelet recovery.
 MOTION THAT CARES
MOTION THAT CARES
 Exclusive soft centrifugation technology that optimizes the platelets and cells separation and concentration, preserving their integrity and viability.
Exclusive soft centrifugation technology that optimizes the platelets and cells separation and concentration, preserving their integrity and viability.
 PRECISE SELECTION
PRECISE SELECTION
 Laminar flow selection system that allows precise PRP selection directly from the tube to the application syringe without platelet activation.
Laminar flow selection system that allows precise PRP selection directly from the tube to the application syringe without platelet activation.
PRODUCES A UNIQUE PRP
VIDEOS





